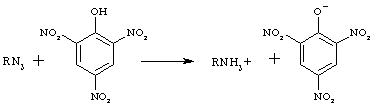 A solution of 0.5 g of the amine in 10 mL of 95% ethanol is prepared. If all of the amine does not dissolve, filter the mixture.
A solution of 0.5 g of the amine in 10 mL of 95% ethanol is prepared. If all of the amine does not dissolve, filter the mixture.Picrates:
From Laboratory Methods in Organic Chemistry Solomon Marmor, 1981 Burgess Publishing Company Minneapolis. p. 500.
"24.6.5 Amines (Tertiary)
Because tertiary amines are incapable of forming sustituted amides or ureas, the usual derivative is some type of salt. Picrates, derivatives of picric acid (2,4,6-trinitophenol), are relatively easy to prepare.
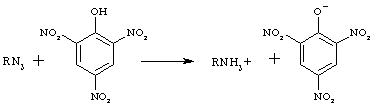 A solution of 0.5 g of the amine in 10 mL of 95% ethanol is prepared. If all of the amine does not dissolve, filter the mixture.
A solution of 0.5 g of the amine in 10 mL of 95% ethanol is prepared. If all of the amine does not dissolve, filter the mixture.From Practical Organic Chemistry, 3rd ed. Arthur I. Vogel, 1966 Longmans, Green and Co Ltd London. p. 422.
"3. Picrates. Picric acid combines with amines to yield molecular compounds (picrates), which usually possess characteristic melting points. Most picrates have the composition 1 mol amine : 1 mol picric acid. The picrates of the amines, particularly of the more basic ones, are generally more stable than the molecular complexes formed between picric acid and the hydrocarbons…
If the amine is soluble in water, mix it with a slight excess (about 25 per cent.) of a saturated solution of picric acid in water (the solubility in cold water is about 1 per cent.). If the amine is insoluble in water, dissolve it by the addition of 2-3 drops of dilute hydrochloric acid (1:1) for each 2-3 ml. of water, then add a slight excess of the reagent. If a heavy precipitate does not form immediately after the addition of the picric acid solution, allow the mixture to stand for some time and then shake vigorously. Filter off the precipitated picrate and recrystallize it from boiling water, alcohol or dilute alcohol, boiling 10 per cent. acetic acid, chloroform or, best, benzene
The method described by Vogel may be a better procedure for heat sensitive amines, since the only heating step is after formation of the picrate and even then, by use of MeOH, temperatures could remain relatively low. Keep in mind that while he describes benzene as the a good choice during recrystalization, it has since been noted to be carcinogenic and is therefore probably not the best choice. The formation of a picrate does not in any way identify a compound as a tertiary amine, since they also form with hydrocarbons, aromatic hydrocarbons, some halogen derivatives and aromatic ethers. Picrates are simply a way of creating a derivative with a characteristic melting point, which is often used in melting point determinations by combination with a known compound (mixed melting points, M.m.p.), watching for depression of melting points.
Ehrlich Test:
W.A. Remers, "Properties and Reactions of Indoles" in: The Chemistry of Heterocyclic Compounds: A Series of Mongraphs, Vol. 25: Indoles Part One W.J. Houlihan ed., 1972, Wiley N.Y. p. 107.
"The formation of a rosindole-type dye provides the basis for the Ehrlich test. This test involves treating an indole with p-dimethylaminobenzaldehyde in hydrochloric acid. A recent study shows rosindole formation to be fast for indoles with no substituent at C(3) and no electron withdrawing groups on the nucleus; slow for indoles substituted at C(3) but containing no free amine in the 3-substituent, and having no electron-withdrawing groups on the nucleus; negative for a 3-substitutuent containing a free amine, for substitution at both C(2) and C(3), and for an electron withdrawing group in the nucleus."
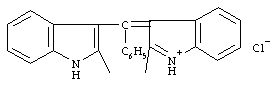
Figure 1: rosindole
Mixed Melting Points:
From Practical Organic Chemistry, 3rd ed. Arthur I. Vogel, 1966 Longmans, Green and Co Ltd London. p. 422.
"I, 17. Mixed melting points. In the majority of cases the presence of a "foreign substance" will lower the melting point of a pure organic compound. This fact is utilised in the so-called mixed melting pointtest for the identification of organic compounds. Let us suppose that an unknown compound X is supplied which is suspected to be o-chlorobenzoic acid since its melting point is 140°. This is tested for by intimately mixing together approximately equal weights of X and an authentic specimen of o-chlorobenzoic acid (A) and determining the melting point